american academy of pediatrics covid vaccine under 12
According to a recent report by the American Academy of Pediatrics AAP and the Childrens Hospital Association CHA. However Moderna announced Thursday that it is seeking emergency use authorization for its.

Think Twice Before Giving The Covid Vax To Healthy Kids Medpage Today
Lee Savio Beers the president of the American Academy of Pediatrics spoke with Fortune about the importance of a vaccine against COVID-19 for the youngest of Americans.
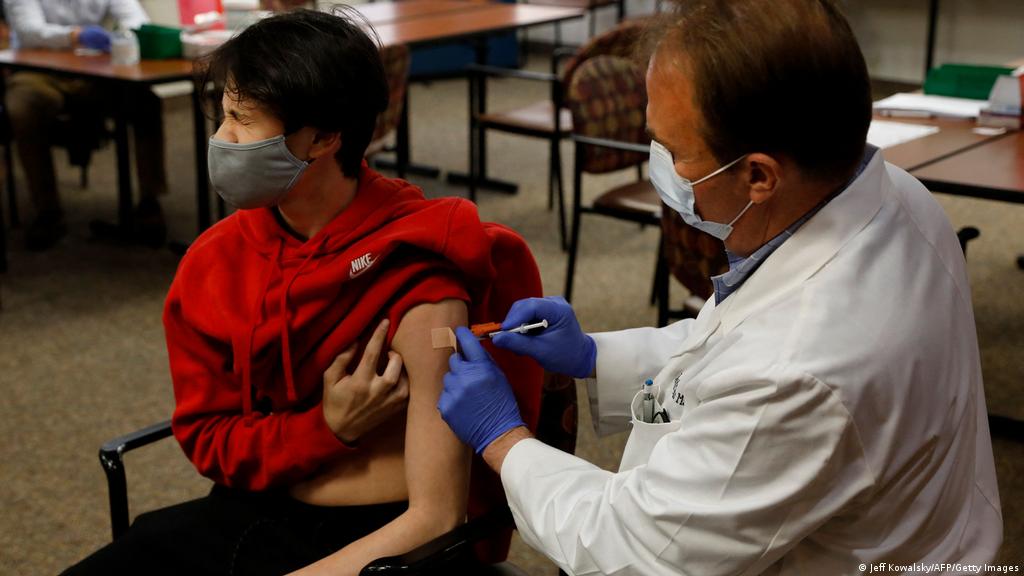
. The American Academy of Pediatrics AAP recommends the following related to coronavirus disease 2019 COVID-19 vaccine in children and adolescents. Download the Full Report 4272022. As COVID-19 vaccines are being administered to anyone age 12 and up Americas youngest children remain the last group that cannot get a vaccination.
The Food and Drug Administration must work aggressively toward authorizing a Covid-19 vaccine for children under age 12 the head of the American Academy of Pediatrics wrote in a letter to Dr. The Centers for Disease Control and Prevention CDC director signed off on boosters for this group Wednesday after an advisory group voted 13-1 in favor and called for a strong recommendation. Adolescents ages 12-15 years can begin receiving COVID-19 vaccine boosters.
Because theyre still growing and developing its critical that these vaccines are evaluated in well-designed and well-conducted clinical trials Children under 5 years would receive doses of 3 micrograms µg. While questions may now arise about the administration of the vaccine off-label for children aged 11 and younger. ShopAAP is the official store of the American Academy of Pediatrics.
This vaccine safety system. Were 67 000 pediatricians committed to the optimal physical mental and social health and well-being for all infants children adolescents and. Maximize protection get all recommended vaccine doses and boosters as soon as possible.
Food and Drug Administration FDA to approve the use of COVID-19 vaccines in children younger than age 12. Lee Savio Beers the president of the American Academy of Pediatrics had to say. The American Academy of Pediatrics and the American Heart Association have endorsed the CDC recommendations and reiterated the potential benefits of COVID-19 vaccination which outweigh the apparent small risk of myopericarditis in children 12 years of age and older4 5 We summarize our findings in 25 adolescents 12-18 years of age with.
All COVID-19 vaccines approved andor granted Emergency Use Authorization EUA by the Food and Drug Administration FDA and vaccines on the Recommended Child and Adolescent Immunization Schedule including co-administered vaccines are monitored through robust FDA and CDC systems that monitor vaccine safety in the United States. FDA authorizes COVID-19 vaccine for adolescents ages 12-15. Cases among Americas youth.
Another recent study showed about 87 of children ages 5-11 years hospitalized with COVID-19 this winter were unvaccinated. Ad The best way to slow the emergence of COVID variants is to get vaccinated. In an interview with NPR on Saturday Dr.
Children under the age of five are the only group left without access to an approved COVID-19 vaccine. NPRs Ailsa Chang speaks with American Academy of Pediatrics President Lee Savio Beers about the mounting pressure to consider emergency use authorization of COVID-19 vaccines for children under 12. The American Academy of Pediatrics AAP is urging the US.
By Joshua Klein A new puberty guide from the American Academy of Pediatrics AAP aimed at 9-12 year olds instructs youth about girls getting erections while normalizing transgender and non-binary characters in order to promote inclusivity. The AAP recommends COVID-19 vaccination for all children and adolescents 5 years of age and older who do not have contraindications using a COVID-19 vaccine authorized for use for their age. Recent research suggests that the Pfizer-BioNTech COVID-19 vaccine authorized for emergency use in children ages 5 to 11 isnt nearly as effective as it is for adolescents or adults.
Currently only the Pfizer COVID-19 vaccine has an emergency use authorization for children 12 and up. Kathryn Lowe one of three co-authors of a new puberty guide USSA News. Food and Drug Administration and the Centers for Disease Control and Prevention.
It is a lower dose than the 10-µg doses used for children ages 5-11 years and 30-µg doses used for those 12 years and older. All purchases directly benefit and support the health and well-being of all infants children adolescents and young adults. Just 28 of children ages 5-11 years are fully vaccinated according to CDC data.
AAP COVID vaccination resources. Judy there are 48 million kids in America under the age of 12 but the timeline for a vaccine for those kids keeps changing. The viral vector vaccine in the United States is given in one dose to people 18 years and older.
AAP calls for completion of clinical trials of the vaccine in younger ages to identify correct dose. Neither the Johnson Johnson or Moderna vaccine have been approved for anyone under age 18. A single booster dose of the Pfizer vaccine is authorized for kids 12 through 17 years old at least 5 months after the second dose of vaccine.
Are running clinical trials on vaccines for children under 12. ITASCA IL -- Today the Food and Drug Administration FDA finalized the full approval of the Pfizer-BioNTech COVID-19 vaccine for ages 16 and older. CDC clinical considerations for administering COVID-19.
On May 12 2021 CDC approved the use of the Pfizer-BioNTech COVID-19 vaccine in persons aged 12-15 years following the vaccines EUA granted by the FDA on May 10th. The American Academy of Pediatrics is encouraged that we may be one step closer to a COVID-19 vaccine being available for children under age 5 pending a rigorous review of Pfizer-BioNTechs scientific data validating its safety and effectiveness by the US. In a New.
Vaccine uptake in children has been slow. The FDA issued an EUA for the Pfizer vaccine for kids aged 5 to 11 on 10292021 followed by ACIP recommendation and CDC approval on 1122021. AAP CDC recommend COVID-19 vaccine for ages 12 and older May 12 2021 In addition to approving the Pfizer-BioNTech vaccine for adolescents the CDC updated its clinical guidance to allow coadministration of vaccines.

Covid Vaccines In Teens And Heart Inflammation What You Need To Know Shots Health News Npr
Pediatricians Besieged By Parents Seeking Coronavirus Shots For Kids Under 12 The Washington Post

Back To School White House Pushes For Kids 12 And Up To Get Covid Vaccine

What Parents Should Know About Covid 19 Vaccines For Kids Under 12

Fact Check How Useful Are Coronavirus Vaccines For Children And Adolescents Coronavirus And Covid 19 Latest News About Covid 19 Dw 06 08 2021
Faq About Pfizer S Covid Vaccine And Kids Shots Health News Npr
Covid Vaccines For Kids Under 12 Expected Midwinter Fda Official Says
/kidcovid-514a2faa59744c7f8e7de7a8cbba640d.jpg)
How Does The Delta Variant Affect Kids
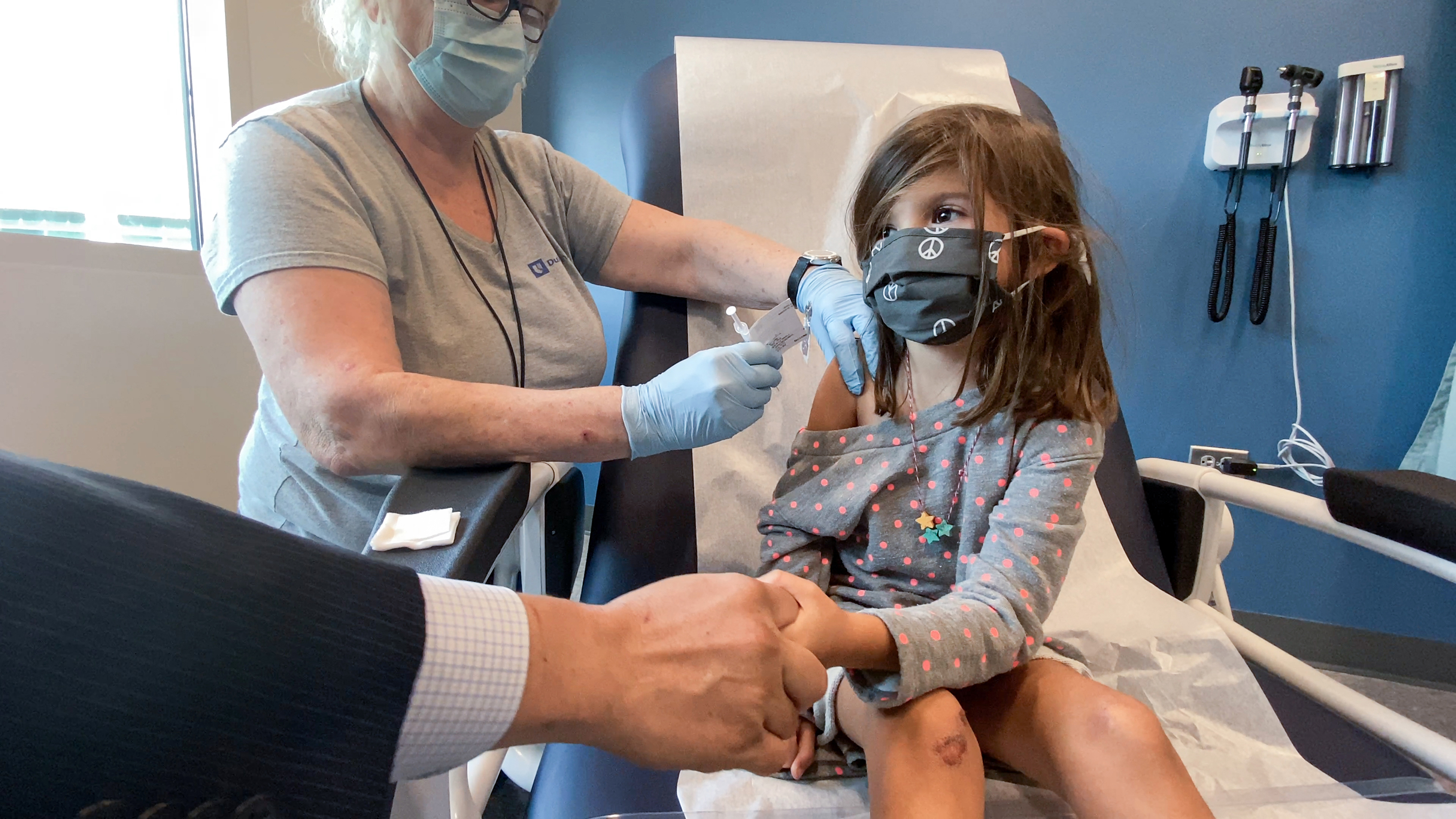
Opinion Why You Should Vaccinate Your 5 To 11 Year Old Cnn
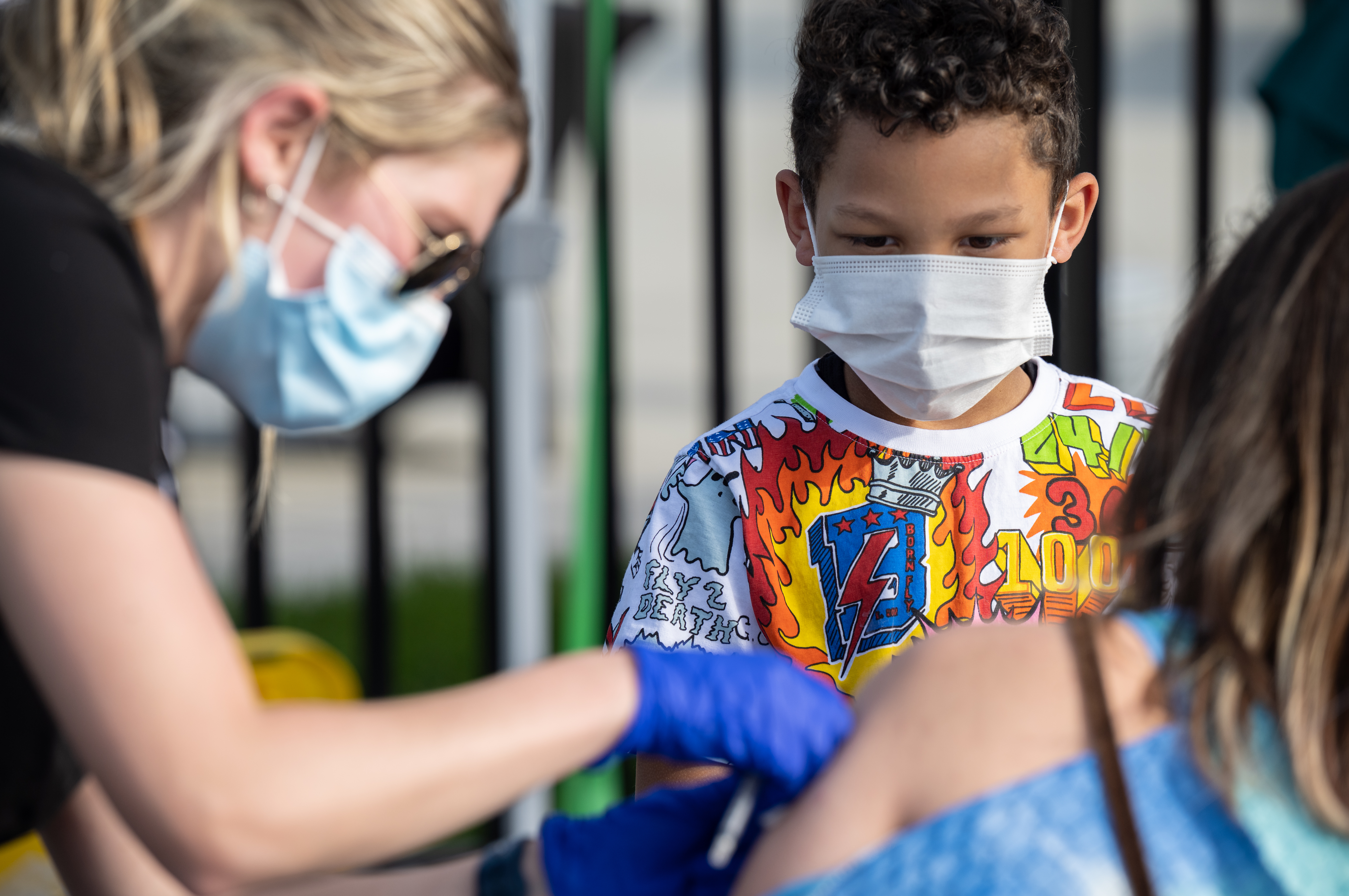
More Children Are Catching Covid 19 But It S Unclear If They Re Getting Sicker Coronavirus Updates Npr
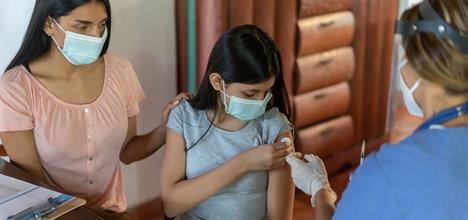
Aap Advises Children And Teens Age 12 And Up Get The Covid 19 Vaccine Healthychildren Org
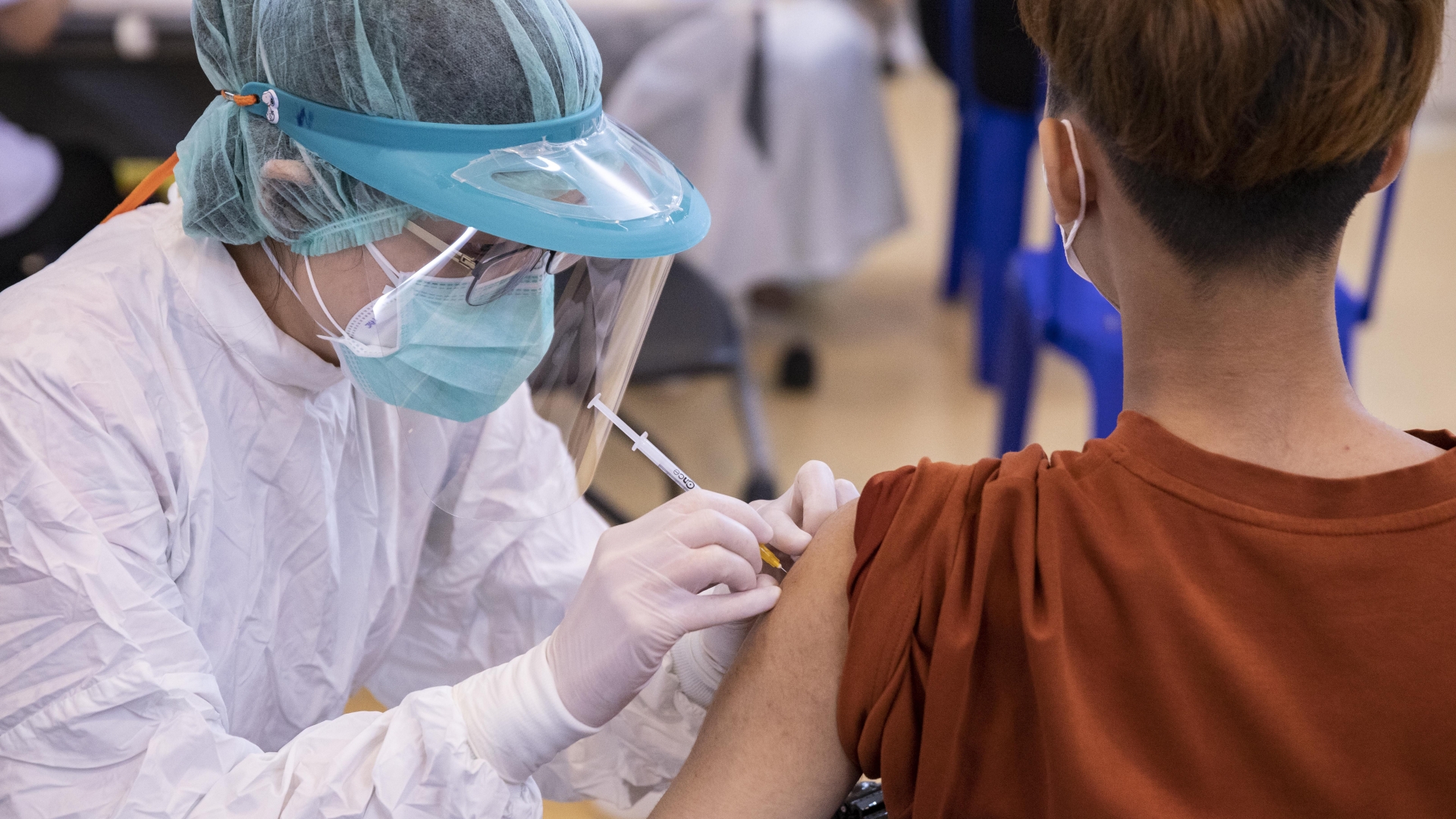
Pfizer Biontech Ask Fda To Authorize Coronavirus Vaccine For Children 5 To 11 The Washington Post

Kids Covid Vaccine Side Effects What To Know

Us Officials Set Stage For Vaccination Campaign For Younger Children Coronavirus The Guardian

Kids Covid Vaccine Side Effects What To Know
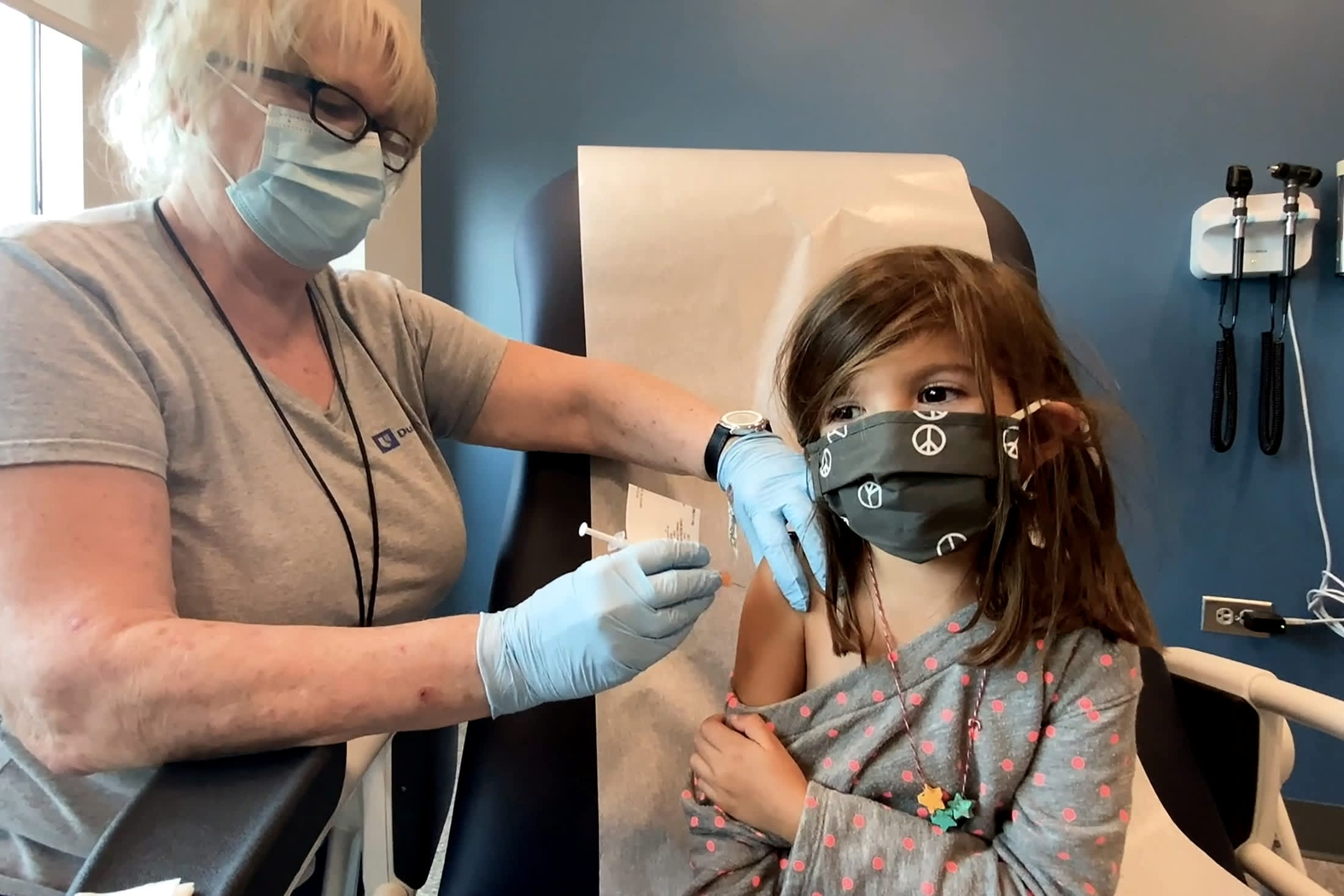
White House Calls On Pediatricians To Help With Rollout Of Kids Covid Vaccine
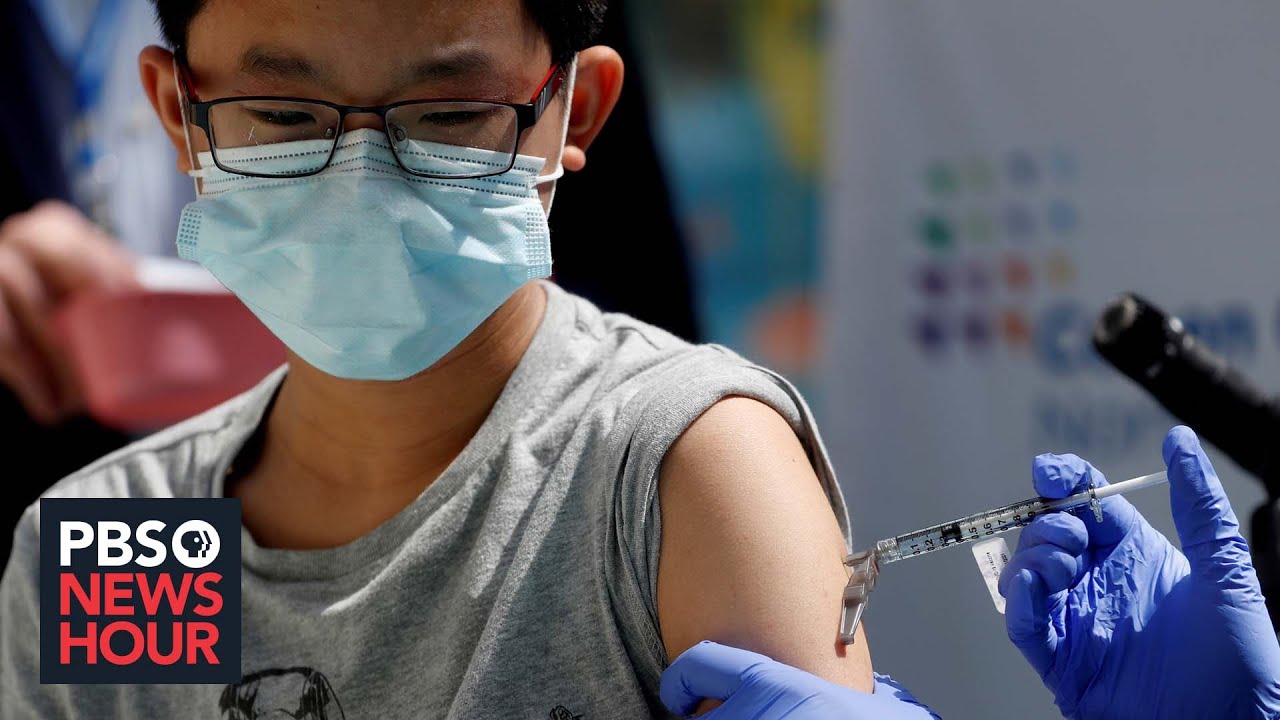
American Academy Of Pediatrics Urges Fda To Approve Covid Vaccines For Children Under 12 Youtube

Pediatricians Want Kids To Be Part Of Covid Vaccine Trials Kaiser Health News
/bogdankosanovic-2d3eb12758ab49749b38568537c56715.jpg)